LASILIX Tablet
ក្រុមហ៊ុនផលិតឱសថ:
Sanofi Winthrop Industrie, France
ក្រុមហ៊ុនចែកចាយឱសថនៅប្រទេសកម្ពុជា:
SANOFI
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Furosemide 40mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
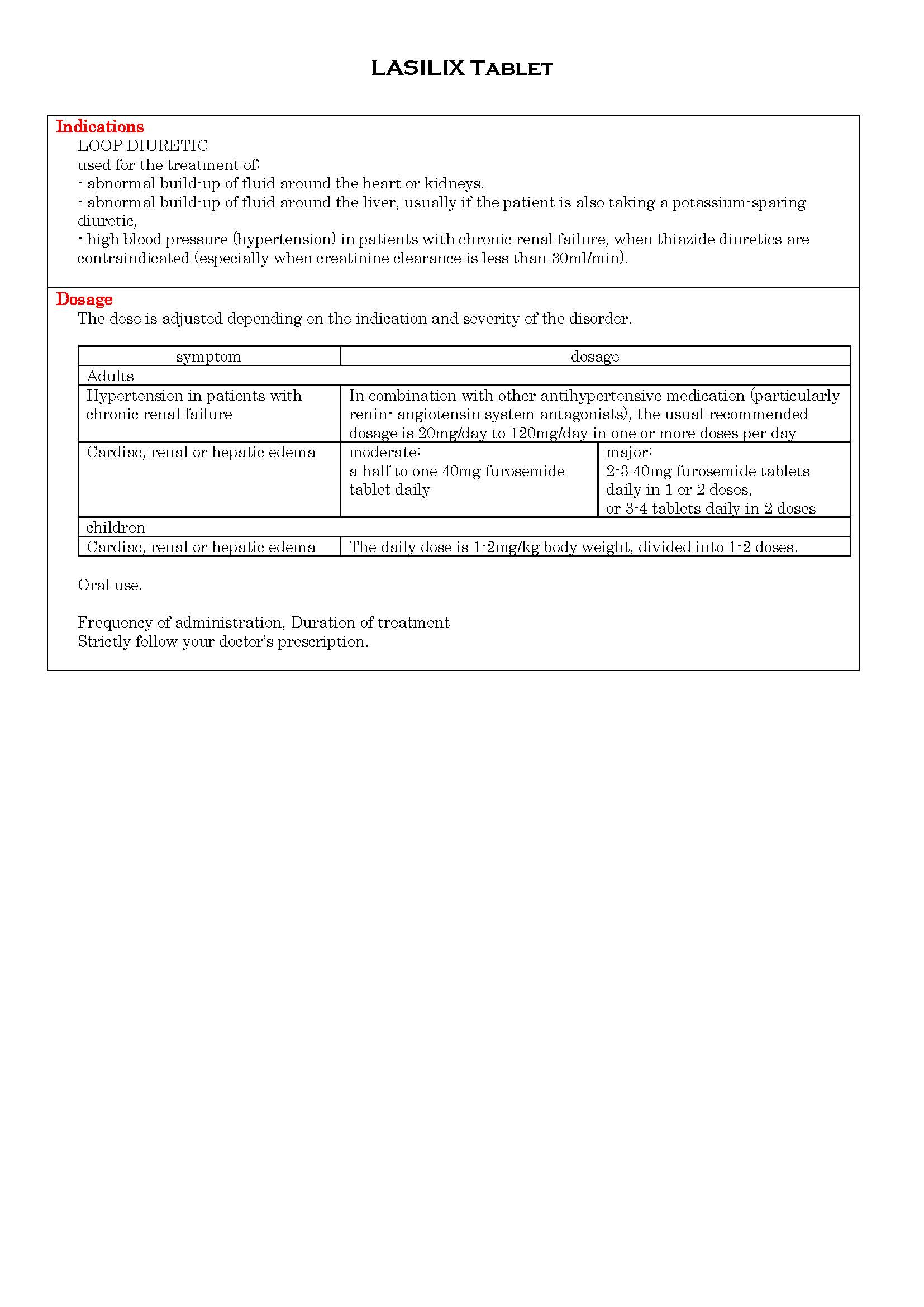
-
ហាមប្រើ
- if you are allergic to furosemide or any of the other ingredients,
- if you have acute renal failure,
- if you have urinary tract obstruction,
- if you have hypovolemia (reduction in total blood volume), or dehydration,
- if you have severe hypokalemia,
- if you have active hepatitis or severe liver failure, particularly in hemodialysis patients and those with severe renal failure,
- if you have hepatic encephalopathy (brain disorderss observed in patients with serious liver disease),
- in general, you should not take this medicine during pregnancy, or in combination with lithium,
- if you are taking another medicine, check that combination with Lasilix is not contraindicated.
-
ផលរំខាន
Side effects
- elevated blood glucose level (amount of sugar in the blood),
- hypokalemia ( low amount of potassium in the blood),
- hyponatraemia (low amounts of sodium in the blood),
- water- electrolyte imbalance which can cause dehydration,
- hypovolemia (low total blood volume in the body), which can lead to postural hypotension (fall in blood pressure when standing up),
- risk or thrombosis (formation of blood clots), particularly in elderly subjects,
- urine retention due to urinary tract obstruction,
- elevated blood cholesterol and triglycerides,
- elevated blood urea and creatinine,
- slight elevation of blood uric acid (amount of uric acid in the blood) which can trigger a gout attack in exceptional cases,
- inpatients with liver failure, possible onset of hepatic encephalopathy (neurological disorder observed in patients with severe liver disease),
- rare cases of allergic or non-allergic skin reactions, itching, hives, occasionally bullous reactions, bullous pemphigoid (skin disorder characterized by the presence of bullae), erythema multiforme, exceptionally: increased sensitivity to the sun, bullous rash with detachment of skin which can rapidly spread over the whole body and be life-threatening (Stevens-Johnson syndrome, toxic epidermal necrolysis),
- allergic reactions:
exceptionally: fever, hypereosinophilia, anaphylactic and/or anaphylactoid reactions (potentially serious allergic reactions),
rarely: purpura (intradermal hemorrhagic lesions), vasculitis (inflammation and impairment of blood vessels); exceptionally, paresthesia (pins and needles),
- digestive disorders: nausea, vomiting, diarrhea,
- exceptionally: liver or pancreas disorders,
- exceptionally: interstitial nephritis (renal impairment)
- rarely: hearing impairment, particularly in patients with renal failure, nephrotic syndrome (renal impairment), or when patients are also taking antibiotics (particularly those in the aminoglycoside group),
- a few rare cases of blood count changes:
neutropenia (reduction in certain white blood cells), thrombocytopenia (abnormally low platelet count), agranulocytosis (insufficient amounts of other blood cell lines), and aplastic anemia.
-
អន្តរប្រតិកម្ម
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, particularly lithium, including medicines obtained without a prescription.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Pregnancy
- As a general rule, this medicine should not be prescribed during pregnancy.
- It should only be used during pregnancy under exceptional circumstances and on your doctor's advice.
- Fetal development should be closely monitored.
Breast-feeding
- Ask your doctor or pharmacist for advice before taking any medicine.
- Breast-feeding should preferably be avoided during treatment with furosemide.
-
ការប្រុងប្រយ័ត្នជាពិសេស
Precautions
Take special care with Lasilix
Special warnings
- Accidental intake of furosemide may cause a reduction in total blood volume with dehydration.
- In patients with partial urinary tract obstruction, use of furosemide may lead to urine retention. Monitoring of urinary function should therefore be initiated, particularly at the start of treatment with furosemide.
- Photosensitivity reactions have been reported with furosemide. If a photosensitivity reaction occurs during treatment, it is recommended to discontinue treatment. If rechallenge is absolutely necessary, it is recommended to protect areas of skin exposed to the sun and artificial UVA rays.
- Use of this medicine is not recommended if you have galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption syndrome (rare hereditary diseases).
Precautions for use
- pre-diabetic conditions and diabetes,
- gout,
- serious liver disease,
- serious kidney disease,
- hypotension.
Special monitoring (blood tests, medical check-up) is required throughout the duration of treatment. If neonates and premature infants are given high doses of this medicine over a long period, they may require monitoring by renal ultrasound.
In elderly subjects with dementia, combination of furosemide with risperidone (a medicine used to treat certain mood or behavior disorders) should be used with caution.
-
សកម្មភាពឱសថ
no listed
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
